If Travelling At Same Speeds Which Of The Following Matter Waves Have The Shortest Wavelength
There we have electron we have proton we have neutron. If travelling at same speeds which of the following matter waves have the shortest wavelength.

8r Review Waves Sound Light 1 What Do Waves Carry Energy 2 What Causes All Waves Vibrations 3 What Types Of Waves Require A Medium Identify An Ppt Download
Multiple Choice Questions Type-II In the following questions two or more options.

If travelling at same speeds which of the following matter waves have the shortest wavelength. The smallest mass then is going to belong to the electron. Iv The two electrons present in the 2s orbital have spin quantum numbers ms but of opposite sign. They are travelling in the same direction but out of phase compared.
15If travelling at same speeds which of the following matter waves have the shortest wavelength. Mass of proton 167271024 g. Identify the pairs which are not of isotopes.
The electromagnetic spectrum ranges from gamma γ radiation which has the shortest wavelength highest frequency and greatest energy to radio waves which has the longest wavelength and lowest frequency and energy. Mass of Alpha particles 4. Ii Alpha particle He 2 iii Neutron.
He 24 mass of proton 41672710 2466810 24 g. Two interferring waves have the same wavelength frequency and amplitude. We have the helium nucleus which is two protons.
If travelling at same speeds which of the following matter waves have shortest wavelength. For this reason the wavelength ratio is the inverse of the frequency ratio. Are solved by group of students and teacher of JEE which is also the largest student community of JEE.
Mass of electron 9110times 10-28gmass of proton 16727times 10-24gmass of neutron 16750times 10-24gmass of Alpha particles. So we can infer from that then that the longest wavelength which is what were looking for will come from the particle that has the smallest mass and of our choices. Asked Aug 18 2018 in Chemistry by Sagarmatha 545k points If travelling at same speeds which of the following matter waves have the shortest wavelength.
The wave with the greatest frequency has the shortest wavelength. If traveling at same speeds which of the following matter waves have the shortest wavelength. So we can infer from that then that the longest wavelength which is what were looking for will come from the particle that has the smallest mass and of our choices.
H e2 4 mass of proton 4167271024 6681024 g. AElectron BAlpha particle CNeutron DProton. If traveling at same speeds which of the following matter waves have the shortest wavelength.
A Electron b Alpha particle He 2 c Neutron d Proton. Mass of neutron 167501024 g. The Questions and Answers of If travelling at same speeds which of the following matter waves have the shortest wavelength.
A Electron b Alpha particle He 2- c Neutron d Proton. Mass of neutron 1675010 24 g. We have the helium nucleus which is two protons.
The smallest mass then is going to belong to the electron. Lambda de-Broglie wavelength h6626times 10-34J_s Plancks constant m mass of particle v speed of particleThus for different particles travelling at same speed the wavelength is inversely proportional to their masses. A Electron b Alpha particle He 2- c Neutron d Proton.
If travelling at same speeds which of the following matter waves have the shortest wavelength. If traveling at same speeds which of the following matter waves have the shortest wavelength. I Electron ii Alpha particle He 2 iii Neutron iv Proton.
More than One Correct Answer Type. Mass of Alpha particles 4. Structure of the atom.
1electron 2alphaparticle He2 3neutron 4proton. Higher is the mass shorter will be the wavelength associated with it. There we have electron we have proton we have neutron.
Twice the frequency means one-half the wavelength. Iv The two electrons present in the 2s orbital have spin quantum numbers ms but of opposite sign. The Questions and Answers of If travelling at same speedswhich of the following matter waves have the shortest wavelength.
I Electron ii Alpha particle He2. Therefore alpha particles having highest mass. Higher is the mass shorter will be the wavelength associated with it.
A Electron b Alpha particle He² c Neutron d Proton. Are solved by group of students and teacher of NEET which is also the largest student community of. Therefore alpha particles having highest mass thus will have shortest wavelength.

It Travelling At Same Speeds Which Of The Following Matter Waves Have The Shortest Wavelength
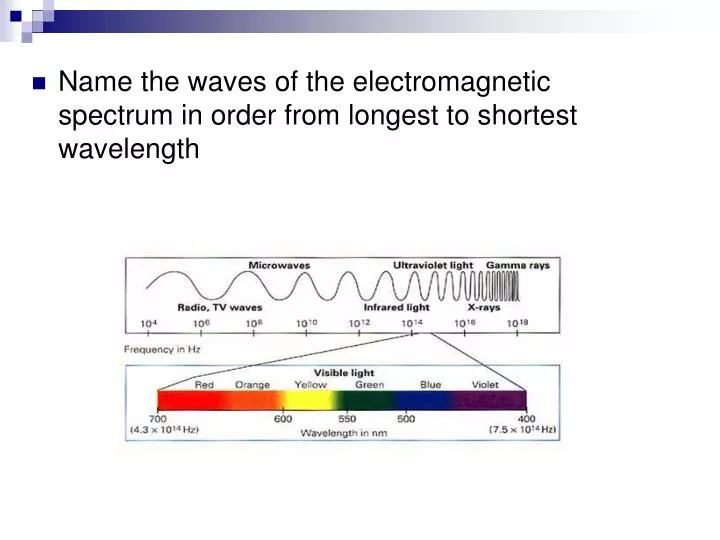
Ppt Name The Waves Of The Electromagnetic Spectrum In Order From Longest To Shortest Wavelength Powerpoint Presentation Id 4390668

If Travelling At Same Speeds Which Of The Following Matter Waves Have The Shortest Wavelength 1 Electron 2 Alphaparticle He2 3 Neutron 4 Proton Edurev Neet Question
Wave Properties Science Quiz Quizizz
Solved If Travelling At Same Speeds Which Of The Following Matter Waves Have The Shortest Wavelength
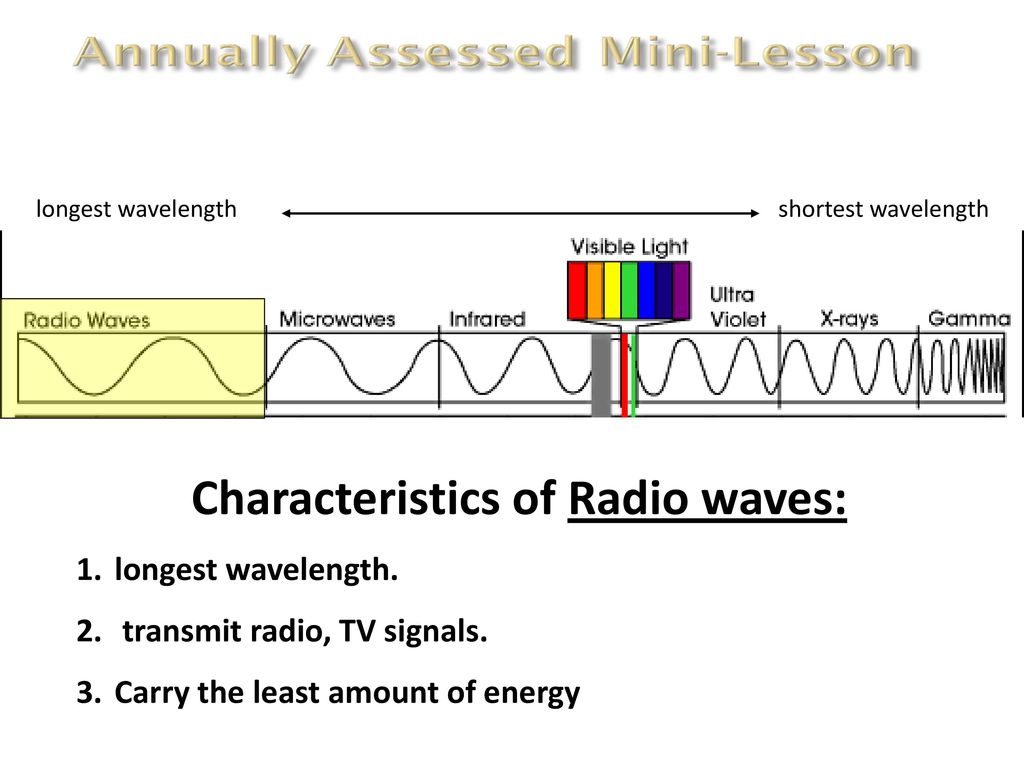
Brainpop Video Waves 4 Minutes Ppt Download
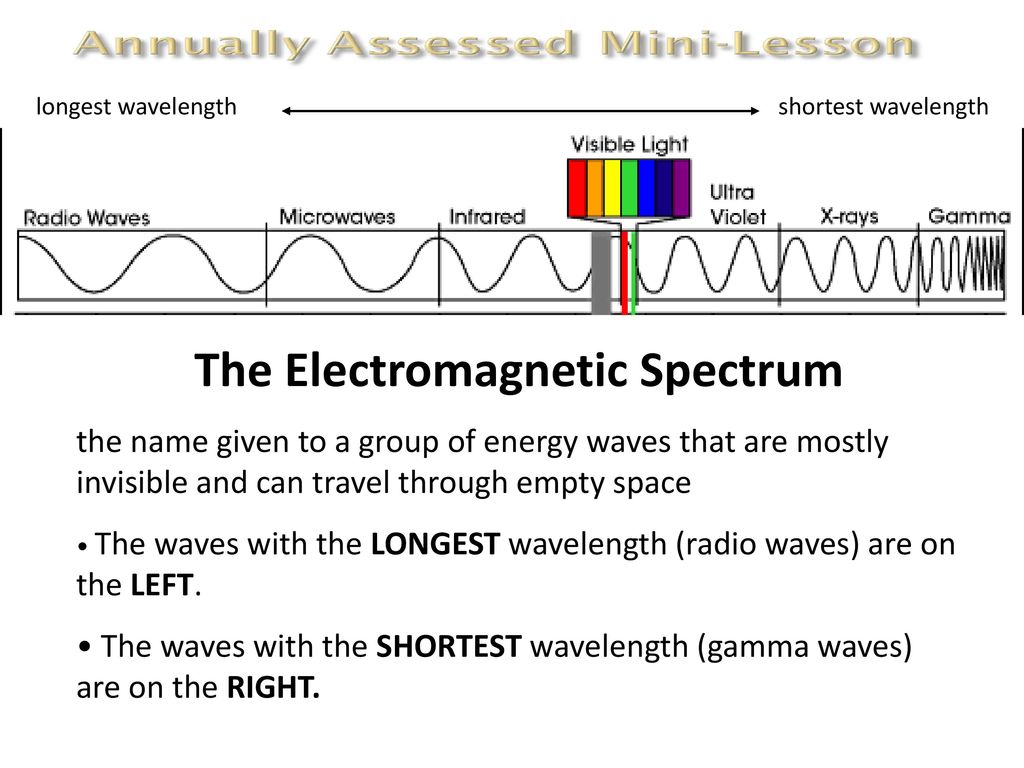
Brainpop Video Waves 4 Minutes Ppt Download
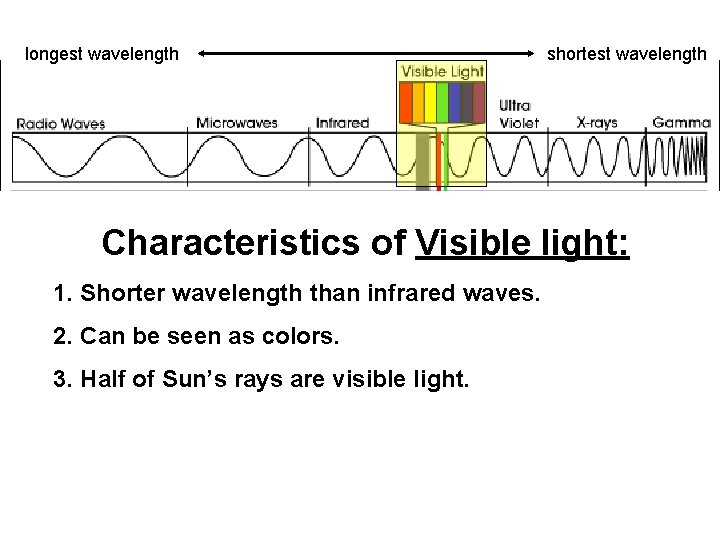
Chemistry Do Now 2 5 19 Directions Take

Electromagnetic Waves Em Waves Waves That Do Not Need A Medium To Travel Through They Can Travel Through A Vacuum Empty Space Examples Of Em Ppt Download

Which Of The Following Matter Waves Will Have The Shortest Wavelength If Travelling With Same Kinetic Energy



Posting Komentar untuk "If Travelling At Same Speeds Which Of The Following Matter Waves Have The Shortest Wavelength"